
THE WATER SUBSTANCE
Earth is often called the Blue Planet for it is the presence of liquid water that sets our home apart in the solar system. Water is a relatively simple but changeable substance. One molecule of water consists of two atoms of hydrogen and one atom of oxygen. This basic molecular structure gives water some unique chemical and physical properties that assure it an extremely important role in maintaining Earth’s habitable environments. Water exists in three quite different forms: solid, liquid and gas. This depends on the temperature and air pressure to which it is exposed.
THE WATER CYCLE
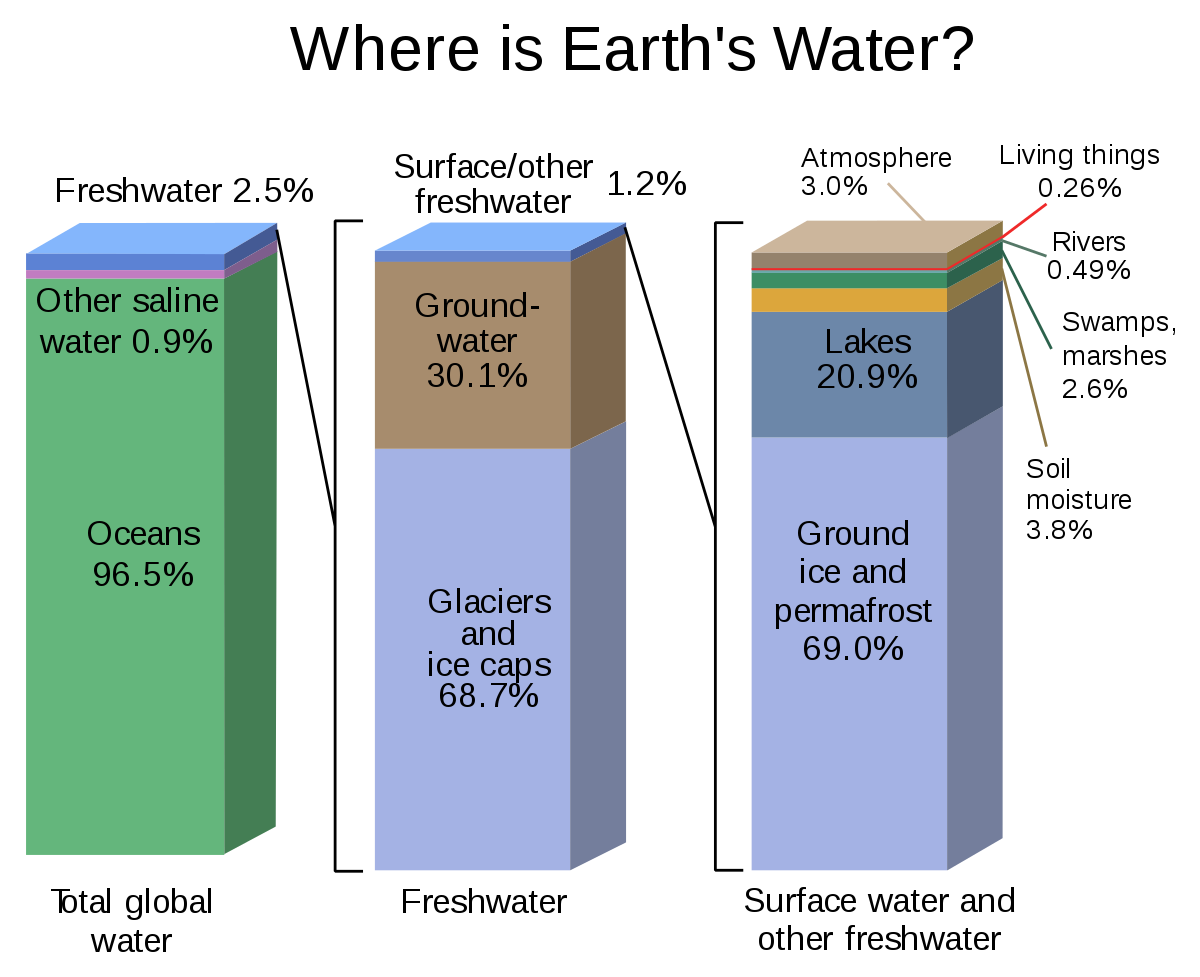
Approximately 97.5 percent of water found on Earth is in liquid form and is found in the oceans. The comparatively small amount of fresh water on the planet is mostly locked up in surface ice or is underground. Only 2.5 percent of Earth’s water is fresh. It is distributed among ice caps, groundwater channels, lakes, vapour, rivers and inside living organisms. Water vapour in the atmosphere only constitutes about 0.001 percent of the total, but has a key role in driving the weather. The water vapour content varies from near zero over dry deserts to about four percent in air saturated with vapour.
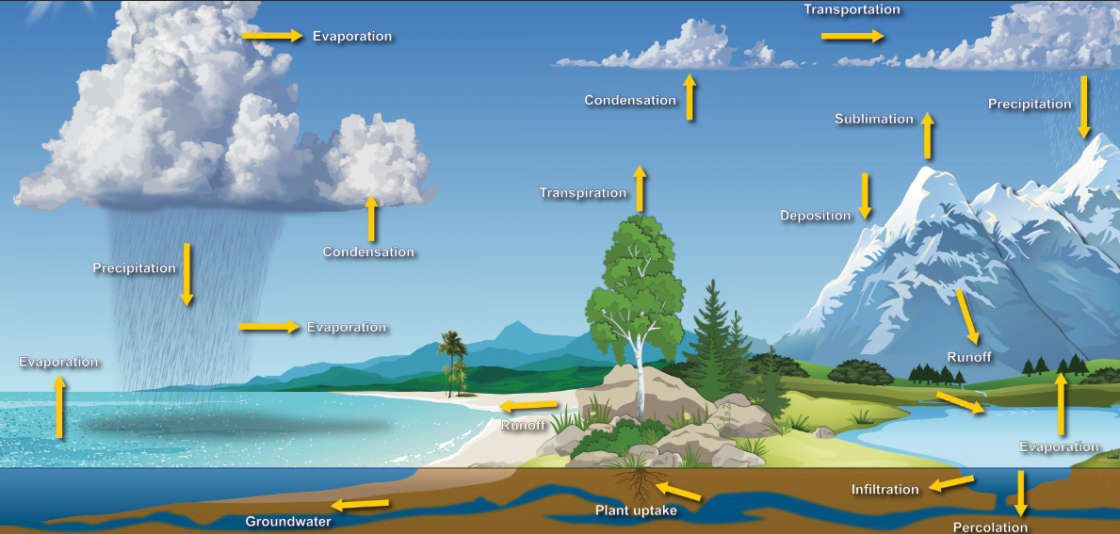
A continuous interchange of moisture between the oceans, land, plants and clouds fuels much of our weather. This process is know as the water cycle. The water cycle is driven by the Sun which causes water to leave the oceans as a result of evaporation. It re-condenses to form small cloud droplets or ice crystals that grow and fall out of clouds, giving us rain and snow. Rivers or groundwater channels carry the water back to the oceans and the cycle continues. Water circulates at a very changeable rate, depending on where it is in the cycle. On average, a water molecule will spend about 10 days in the atmosphere but 10,000 years as deep groundwater polska-ed.com. Residence time in the oceans is longer still, at about 37,000 years.
HUMIDITY
Humidity is the measure of the water vapour content in the atmosphere. Absolute humidity is the mass of water vapour in a given volume of air measured in grams per cubic metre. Specific humidity is similar but is expressed in grams of water per kilogram of air. Relative humidity, on the other hand, is the amount of water vapour in the air at a given temperature expressed as a percentage of the maximum amount of vapour that the air can hold at that temperature. In forecast, reference is made to relative humidity. If the relative humidity is 100 per cent, the air is saturated. If it lies between 80 and 99 per cent, the air is said to be moist and the weather is humid or clammy. When relative humidity drops to 50 per cent, the air is dry. Figures as low as 10 per cent have been recorded over hot deserts.
Humidity depends upon the temperature of the air. At any given temperature, there is a limit to the amount of moisture that the air can hold. When this limit is reached, the air is said to be saturated. Cold air can hold only relatively small quantities of vapour before becoming saturated but this amount increases rapidly as temperatures rise. This means that the amount of precipitation obtained from warm air is generally greater than that from cold air.


0 comments
Write a comment